Synvisc one 1 GENZYME 6 ml syringe
For warnings, precautions for use and contraindications, please consult the instructions for use.
Please send us your prescription with your order.
Description Synvisc one 1 GENZYME 6 ml syringe
Synvisc one (Hylan GF 20) is a viscoelastic solution of hyaluronic acid recommended to relieve pain caused by osteoarthritis (a condition that causes "wear and tear" on the knee joint). Synvisc one (Hylan GF 20) has been shown to be effective when administered in all stages of joint disease. Supplementation with Synvisc one (Hylan GF 20) is a treatment that reduces pain and discomfort and thus increases the range of motion of the joint.
Description of Synvisc one
Synvisc one (Hylan GF 20) is a sterile, non-pyrogenic elastovisous fluid that contains hylans. Hylans are derivatives of hyaluronan (sodium hyaluronate) and are made up of repeating units of N-acetylglucosamine and sodium glucuronate. Synvisc one (Hylan GF 20) is biologically similar to hyaluronan. In vitro studies have shown that Synvisc one (Hylan GF 20) protects cartilage cells against certain physical and chilic lesions. Pain relief can last up to 6 months.
Directions for use of Synvisc one
Intra-articular route. Reserved for adults. The usual recommended dose is one injection into the knee joint. It is important that this medical device is used as directed by your doctor. Synvisc one (Hylan GF 20) should only be administered intraarticularly and by a doctor.
Composition of Synvisc one
Viscoelastic solution, hyaluronic acid. Each ml of sterile, pyrogen-free elastovis liquid contains 8 mg of hylan. Excipient with known effect: disodium hydrogen phosphate, sodium chloride, sodium dihydrogen phosphate monohydrate and water for injection.
Precautions for using Synvisc one
Keep out of reach of children. Never use Synvisc one (Hylan GF 20) if you are allergic to any of the constituents, if you have a skin condition at the injection site, if your joint is infected or inflamed.
Synvisc one overview
Box of 1 syringe of 6 ml.
Our advice from pharmacy experts
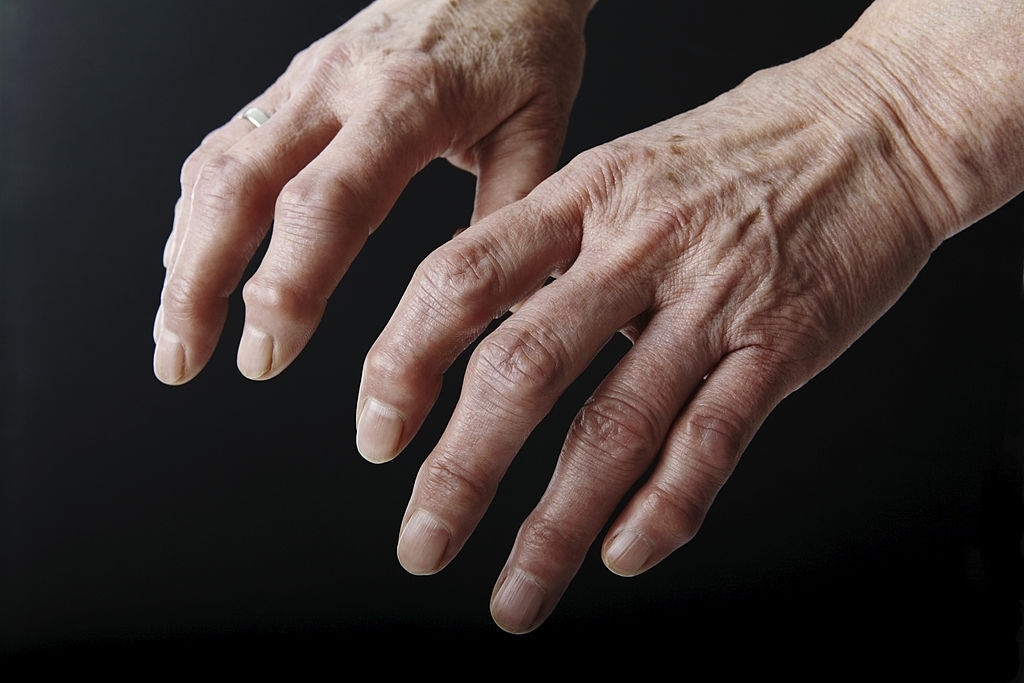
What is a joint made of ?
A healthy joint is made up of cartilage and synovial fluid, which protect the bones from impact and friction.
In an arthritis joint, the cartilage crumbles and the synovial fluid can no longer play its protective role. There is then a friction between the bones which causes pain, joint stiffness and swelling.
The injection of Synvisc-One replaces the arthritic synovial fluid with a natural substance forming a cushion which protects and lubricates the joint.
In the osteoarthritis knee, synovial fluid (also called "synovia") can leak out. When this happens, the knee loses its protective, lubricating pad.
Synvisc-One replaces arthritis synovial fluid to relieve pain and improve the ability of the joint to act as a natural shock absorber.
Warnings on pain relievers
Attention, the drug is not a product like the others. Read the package leaflet carefully before ordering. Keep medicines out of the reach of children. If symptoms persist, seek the advice of your doctor or pharmacist. Beware of incompatibilities on your current products.
- Please inform your online pharmacist of the treatments in progress in order to identify any incompatibilities. The order validation form contains a personalized message field provided for this purpose.
- Click here to find the leaflet for this medication on the website of the National Agency for the Safety of Medicines and Health Products.
- Pharmacovigilance : Declare one or more undesirable effect (s) linked to the use of a drug.