NOTICE
ANSM - Updated on : 11/01/2024
Product name
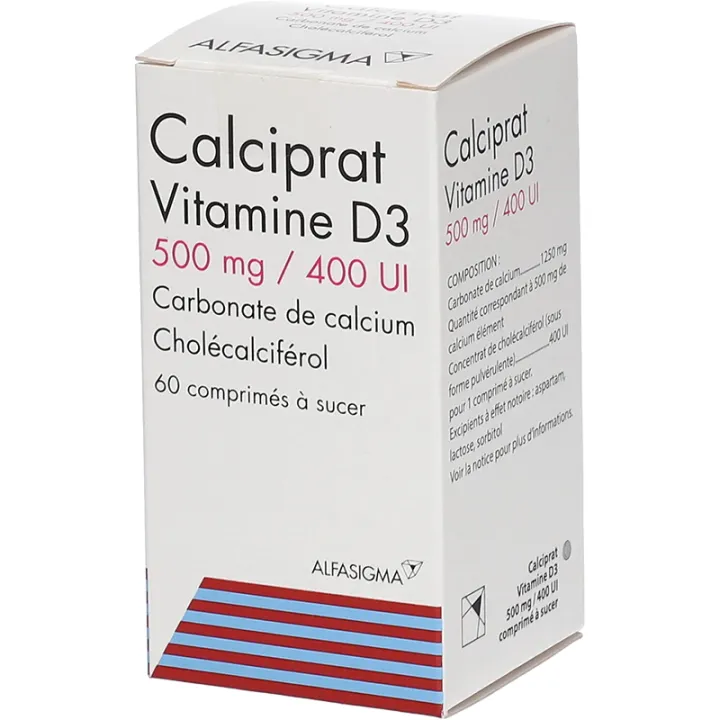
CALCIPRAT VITAMIN D3 500 mg/400 IU, chewable tablet
Calcium/Cholecalciferol
Box
Please read this leaflet carefully before taking this medicine. It contains important information for your treatment.
If you have any further questions, please ask your doctor or pharmacist.
- Keep this leaflet as you may need to read it again.
- If you need more information or advice, ask your pharmacist.
- If symptoms worsen or persist, consult your doctor.
- If you notice any side effects not mentioned in this leaflet, or if you experience any of the effects mentioned as serious, please tell your doctor or pharmacist.
Leaflet summary
In this leaflet :
1. WHAT IS CALCIPRAT VITAMIN D3 500 mg/400 IU, chewable tablet AND WHAT IS IT USED FOR?
2. WHAT DO I HAVE TO KNOW BEFORE TAKING CALCIPRAT VITAMIN D3 500 mg/400 IU, Sucking Tablet?
3. HOW DO I TAKE CALCIPRAT VITAMIN D3 500 mg/400 IU, Sucking Tablet?
4. WHAT ARE THE POSSIBLE SIDE EFFECTS?
5. HOW CAN I USE CALCIPRAT VITAMIN D3 500 mg/400 IU, Sucking Tablets?
6. ADDITIONAL INFORMATION
1. WHAT IS CALCIPRAT VITAMIN D3 500 mg/400 IU SUCKABLE TABLET AND WHAT IS IT USED FOR?
Pharmacotherapeutic class
VITAMIN D-CALCIUM INTAKE
(A: Digestive system and metabolism - Active on calcium balance).
Therapeutic indications
This drug is indicated:
- in elderly patients suffering from calcium and vitamin D deficiency,
- in association with osteoporosis treatments when calcium and vitamin D intake is insufficient.
2. WHAT DO I HAVE TO KNOW BEFORE TAKING CALCIPRAT VITAMIN D3 500 mg/400 IU, chewable tablet?
List of information required before taking the medicine
Not applicable.
Contraindications
Never take CALCIPRAT VITAMIN D3 500 mg/400 IU in the following cases:
- hypersensitivity to vitamin D or any of its constituents,
- abnormally high levels of calcium in the blood (hypercalcemia),
- exaggerated elimination of calcium in the urine (hypercalciuria),
- kidney stones (calcium lithiasis),
- phenylketonuria (presence of aspartam).
IF IN DOUBT, ASK YOUR DOCTOR OR PHARMACIST FOR ADVICE.
Precautions for use; special warnings
Be careful with CALCIPRAT VITAMIN D3 500 mg/400 IU:
Precautions for use
Use with CAUTION in case of:
- of prolonged treatment, it is necessary to regularly check the amount of calcium in the blood and eliminated in the urine (calciuria). Depending on the results, your doctor may decide to reduce or even discontinue your treatment,
- additional administration of high doses of calcium and vitamin D, under strict medical supervision.
- In the case of combined treatment with sodium fluoride or biphosphonates, it is advisable to wait two hours between taking these drugs and CALCIPRATD3, and in the case of treatment with a tetracycline antibiotic, it is advisable to wait three hours.
- in case of sarcoidosis or renal insufficiency.
IF IN DOUBT, ASK YOUR DOCTOR OR PHARMACIST FOR ADVICE.
Interactions with other drugs
Taking or using other drugs
TO AVOID INTERACTIONS BETWEEN MEDICATIONS, IT IS ESSENTIAL TO REPORT ANY OTHER CURRENT TREATMENT TO YOUR DOCTOR OR PHARMACIST, especially digitalis, tetracyclines, vitamin D, sodium fluoride, biphosphonates, diuretics, antiepileptics, corticoids.
Interactions with food and beverages
Not applicable.
Interactions with herbal products or alternative therapies
Not applicable.
Use during pregnancy and breast-feeding
Pregnancy and breast-feeding
In the event of pregnancy, do not exceed 1500 mg calcium and 600 IU CALCIPRAT D3 per day: one tablet per day.
Ask your doctor or pharmacist for advice before taking any medication.
Sportsmen
Not applicable.
Effects on ability to drive and use machines
Driving vehicles and using machines
No particular effect.
List of notable excipients
List of excipients: aspartam.
3. HOW TO TAKE CALCIPRAT VITAMIN D3 500 mg/400 IU, suckable tablet?
Instructions for proper use
Not applicable.
Dosage, Method and/or route(s) of administration, Frequency of administration and Duration of treatment
Dosage
2 tablets per day.
Method of administration
Oral administration.
Suck tablet followed by a glass of water.
Duration of treatment
In all cases, adhere strictly to your doctor's prescription.
Symptoms and instructions in case of overdose
If you have taken more CALCIPRAT VITAMIN D3 500 mg/400 IU than you should have:
If you take too much CALCIPRAT D3, you may experience the following symptoms:
- nausea, vomiting, intense thirst, constipation.
If such effects occur, inform your doctor immediately, who will take the necessary measures.
In the event of prolonged overdosage, calcifications may appear in vessels or tissues.
Instructions in case of missed dose(s)
If you forget to take CALCIPRAT VITAMIN D3 500 mg/400 IU:
If you miss a dose, do not take two doses at once,
Risk of withdrawal syndrome
Not applicable.
4. WHAT ARE THE POSSIBLE SIDE EFFECTS?
Description of side effects
Like all medicines, CALCIPRAT VITAMIN D3 500 mg/400 IU is likely to have undesirable effects, although not everyone is subject to them:
- Constipation, diarrhea
- Epigastric pain, bloating,
- Nausea.
- Increased calcium levels in the blood or urine in some cases.
Reporting side effects
If you experience any side effects, please tell your doctor, pharmacist or nurse. This also applies to any side effects not mentioned in this leaflet. You can also report adverse reactions directly via the national reporting system: Agence nationale de sécurité du médicament et des produits de santé (ANSM) and the network of Centres Régionaux de Pharmacovigilance - Website: https://signalement.social-sante.gouv.fr/
By reporting adverse reactions, you are helping to provide more information on drug safety.
5. HOW TO CONSERVE CALCIPRAT VITAMIN D3 500 mg/400 IU, sucking tablet?
Keep out of the reach and sight of children.
Expiration date
Do not use CALCIPRAT VITAMIN D3 500 mg/400 IU after the expiration date stated on the box.
Storage conditions
No special storage precautions.
If necessary, warnings against certain visible signs of deterioration
Medicines must not be disposed of down the drain or with household waste. Ask your pharmacist what to do with unused medicines. These measures will help protect the environment.
6. FURTHER INFORMATION
Complete list of active ingredients and excipients
What does CALCIPRAT VITAMIN D3 500 mg/400 IU contain?
The active ingredients are:
Cholecalciferol* concentrate
Quantity corresponding to Cholecalciferol .......................................................................................... 400 U.I
Calcium carbonate .................................................................................................................... 1250 mg
Quantity corresponding to elemental calcium ........................................................................................ 500 mg
For one chewable tablet.
*In powder form
Other components are:
Xylitol, povidone (K 30), menthol, aspartam, mint flavor, talc, magnesium stearate, lactose.
Pharmaceutical form and contents
What is CALCIPRAT VITAMIN D3 500 mg/400 IU and what does it contain?
This medicine takes the form of a chewable tablet. Box of 60.
Name and address of the marketing authorization holder and of the manufacturing authorization holder responsible for batch release, if different
Holder
ALFASIGMA FRANCE
Operator
ALFASIGMA FRANCE
Manufacturer
Laboratoires MACORS
Rue des caillottes
89000 AUXERRE
Product names in member states of the European Economic Area
Not applicable.
Date of approval of the package leaflet
This leaflet was last approved on {date}.
MA under exceptional circumstances
Not applicable.
Internet information
Detailed information on this product is available on the Afssaps (France) website.
Information reserved for healthcare professionals
Not applicable.
Other
Not applicable.